For the experimenter, choosing the right CO2 incubator can do more with less. Advances in research in cell culture applications are taking place as never before. Cultured cells are used in vaccine and pharmaceutical production, toxicity testing, regenerative medicine, cell therapy, new cancer models, immunology, neurology, and the like. In these increasingly sensitive applications, the normal expression of proteins and health status of cells is particularly important.
Although the surface of the CO2 incubator looks similar, the culture effect may be quite different. What is the reason?

Today, Xiaobian will give you a few tricks to learn and use, to help you choose a carbon dioxide incubator.
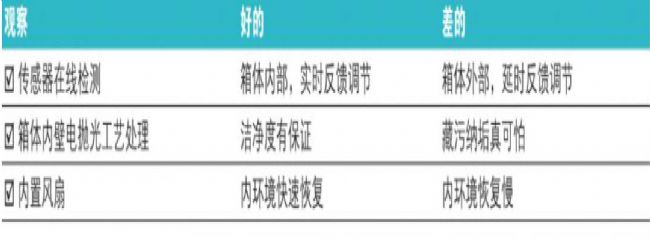
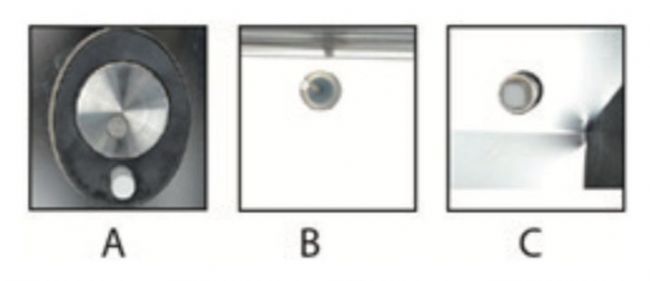
✔Online sensor design, all Thermo ScientificTM CO2 incubators contain high quality intracavity sensors. The sensor is placed in the incubator to ensure that the measurement accurately reflects the environment in which the cells are cultured.
For example: (A) thermal conductivity CO2 sensor and relative humidity compensation (B) dual temperature probe (C) zirconia O2 sensor.
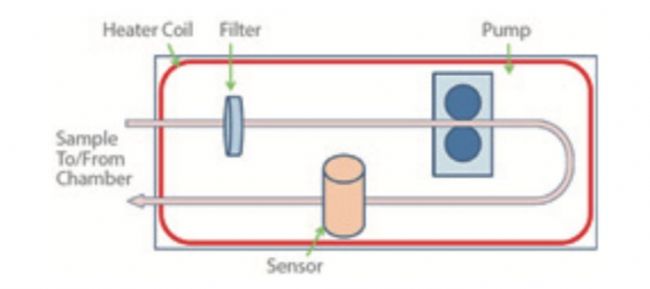
✘ Bypass sensor design, the sensor is placed outside the chamber of the incubator, through additional equipment, including a filter, piping, pump, and a separate heater. Small samples of air, distance traveled and different environments mean that conditions do not match the experience of indoor cells.
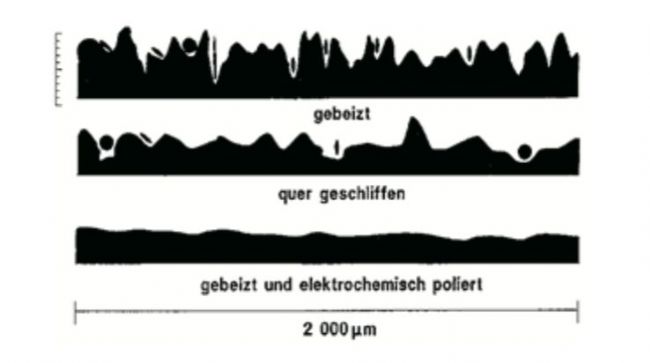
✔The Thermo ScientificTM CO2 incubator is electropolished on the inside of the cabinet for easy cleaning and cleanliness.
✘The surface of the inside of the box that has not been electropolished is filled with various gullies, which is easy to hide.
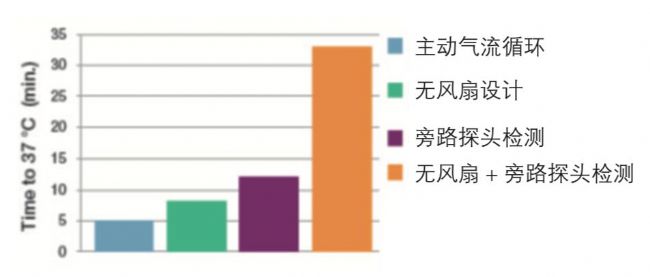
✔The Thermo ScientificTM CO2 incubator is equipped with a fan, plus active air circulation, the environment inside the cabinet can be quickly realized.
ç®± There is no fan-designed cabinet. If the probe is bypassed, the feedback adjustment time is even more than half an hour.

RT-PCR Test Reagent Kit Of COVID-19(ORF1ab, N)
The RT-PCR Test Reagent Kit Of COVID-19(ORF1ab, N) is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper and lower respiratory specimens (such as anterior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal swabs, oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate) from individuals suspected of COVID-19 by their healthcare provider.
Results are for the identification of SARS-CoV-2 RNA.The SARS-CoV-2 RNA is generally detectable in upper and lower respiratory specimens during the acute phase of infection.
Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infective status. The agent detected may not be the definite cause of disease. Positive results do not rule out bacterial co-infection with other viruses.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. The COVID-19 RT-PCR Detection Kit is intended for use by qualified trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.
RT-PCR testing reagent kit,RT PCR test reagent,Covid-19 PCR Diagnostic reagent,RT PCR reagent kit,real time PCR test reagent
Shenzhen Uni-medica Technology Co.,Ltd , https://www.unimedicadevice.com