Medical Network January 21st In recent years, the national medical policy has been frequent, especially with the continuous advancement of the new medical reform policy, the two-vote system, zero drug addition, drug registration, consistency evaluation and other policies have come to the fore Let the industry face new opportunities and challenges.
In fact, there are still a number of policies that are gradually changing the pattern of China's drug market. That is, the system that has begun to advance in 2015 and is now gradually producing outstanding results for review and approval and encouraging innovation. According to preliminary incomplete statistics, at least 100 representative new drugs have been approved since 2016, and the market size of more than 80 imported varieties listed on the global market is close to 100 billion US dollars, and the domestic market space is expected to be 100 billion yuan.
The new drug is listed on the market
In recent years, not only trains, but also new drugs have been approved for listing.
The number of 100 approved new drugs compiled by the author in 2016-2018 was 6, 43 and 50 respectively. It can be seen that the effect of priority review gradually appeared, and the approved new drugs were greatly accelerated. (See the list at the end of the article for details)
From the field of treatment of 100 drugs, the number of oncology drugs is up to 34, and the whole body is resistant to infection (mainly for hepatitis C and AIDS), cardiovascular system, digestive system and metabolic drugs (with diabetes The main) also has more varieties.
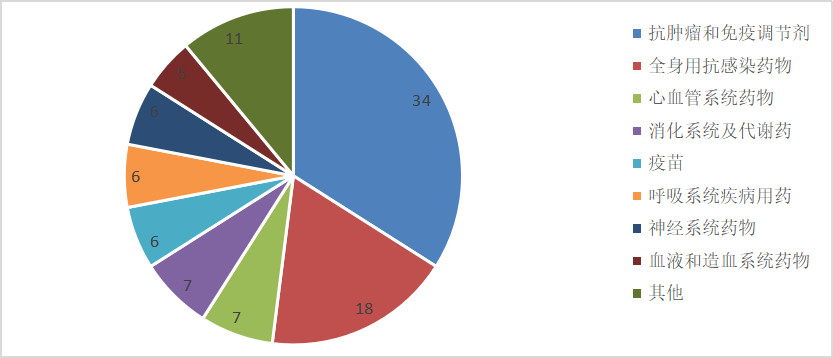
Note: According to the public information, for reference.
From the perspective of the review and approval cycle, most of the new drugs were approved in the form of priority review; there were 3 new drugs with a total approval period of less than 6 months, and many drug approval cycles were less than 2 years.
Judging from whether domestically approved drugs are domestic or imported, most of the new drugs approved in recent years are mainly imported, with 83 imported drugs and only 17 domestically produced drugs. The domestic market for these drugs is expected to be more than 100 billion yuan.
Most of the 83 imported drugs have been listed globally. According to incomplete statistics, the total global sales of these drugs in 2017 is close to 90 billion US dollars.
From the perspective of manufacturers' distribution, Johnson & Johnson, Merck, Novartis, Boehringer and other companies have received more than 7 new drugs in China in recent years, and most of them are 1-2.
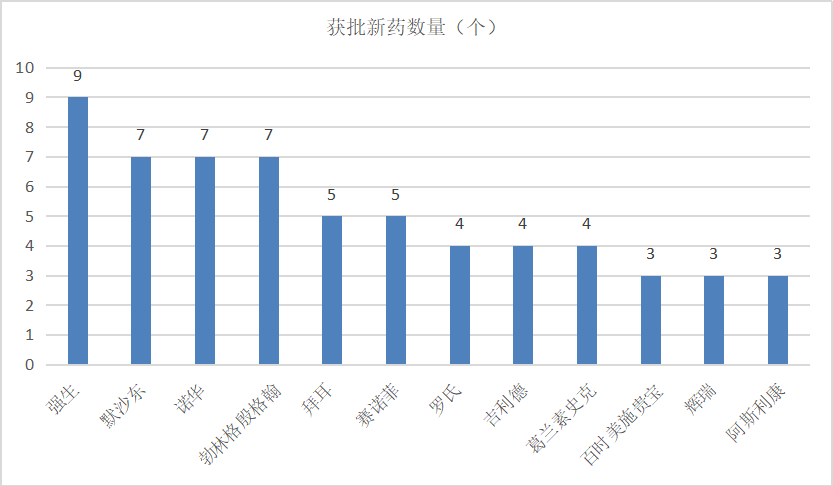
Note: According to the public information, for reference.
This is inseparable from the priority review and approval policy that the country began to strengthen in 2016. The former State Food and Drug Administration issued the "Opinions on Resolving the Backlog of Drug Registration Applications for Priority Review and Approval", and included a variety of situations in the priority review, including the prevention and treatment of AIDS, tuberculosis, viral hepatitis, rare diseases, and malignant drugs. And the application for registration of the “life-saving medicine†for children and the elderly.
Since then, a large number of drugs have been reported and approved through the priority review and approval process. By the end of 2018, a total of more than 30 batches of 770 registration applications were included in the priority review process.
â– Encourage innovation, new drugs gradually integrate with the international market
* Encourage frequent innovation policies
Since 2015, the policy of drug research and development has been oriented, and the time for approval of new drugs has been greatly shortened. Among them, the obvious difference is that the time difference between foreign new drugs entering China has been greatly shortened. Relevant policies are mainly reflected in the priority review and approval + speeding up the domestic listing of imported drugs + joining the ICH + drug listing licensor + chemical drug registration classification changes: survival of the fittest, encouraging innovation, speeding up the review, reducing the backlog of registration, and international standards.
Since 2015, many domestic departments have issued a number of policies to encourage drug R&D innovation and encourage drug approval and approval. There are programmatic documents such as the State Council’s review and approval system for reforming pharmaceutical medical devices on August 18, 2018. The opinion of Guofa [2015] No. 44 (commonly known as No. 44), as well as specific policy documents such as the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council on October 8, 2017 on "Deepening the Review and Examination and Approval System Reform and Encouraging Drug Medical Device Innovation "Opinions", there are also supporting measures such as CNDA, the joint publication of the Health and Health Commission, "Notice on the Approval and Approval of Priority Drug Registration and Approval (No. 23 of 2018)", CNDA's "Overseas Clinical Trial Data Technology for Receiving Drugs" Guiding Principles, etc.
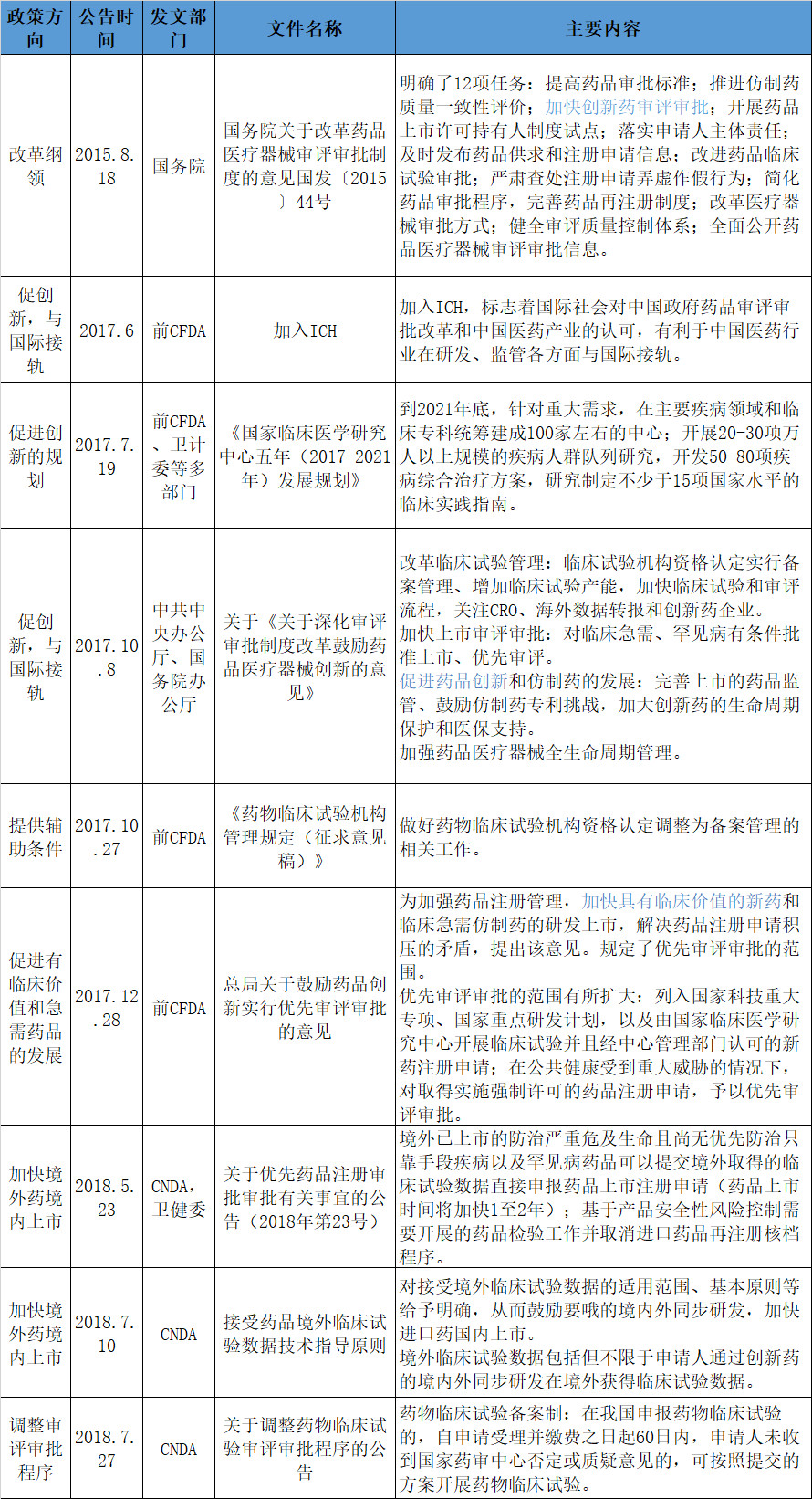
Note: According to the public information, for reference.
* China's new drug listing gradually shortens the "time difference" between domestic and overseas listings
In the past, due to various reasons, there were “time difference†problems in the overseas listing of new drugs in the country, and some drugs with significant curative effects could not benefit domestic patients in time. The data shows that in the past ten years, there have been 415 new drugs listed in the United States, the European Union, and Japan, of which 76 have been listed in China, and 201 are in clinical trials or declarations in China.
In the past two years, the State Drug Administration has continuously simplified the procedures for the approval of overseas listed new drugs. The above-mentioned policies have been gradually implemented, especially the promotion of limited review and approval has contributed to the rapid increase in drug imports.
At present, the effect of the priority review and approval policy has begun to appear, and the listing of new foreign drugs has been accelerating. In the short period of two months from April to June this year, seven treatments, such as the nine-valent human papillomavirus (HPV) vaccine, the sophos-bruze-wet compound preparation, and the PD-1 antibody drug Navulitis monoclonal antibody injection, were seriously endangered. New overseas medicines for life diseases have been approved for listing in China through rapid review. These drugs are urgently needed drugs for the treatment of tumors, hepatitis C and rare diseases.
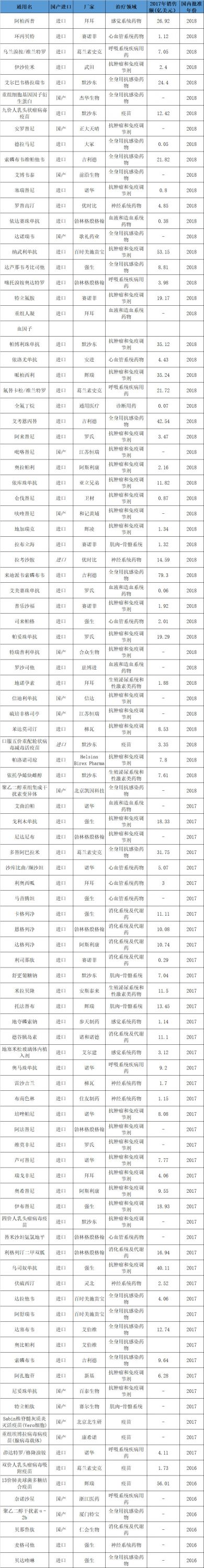
Attachment: List of major new drugs approved for domestic listing in the past three years
Halal Empty Capsule,Halal Empty Gelatin Capsules,Empty Capsule Shell With Halal,Halal Shell Empty Gelatin Capsules
Ningbo Jiangnan Capsule Co., Ltd. , https://www.ningbocapsule.com