"Medical accident, due to misuse leads to death row the top three causes of medical things."
- Washington Post [2013]
The emergence of new types of medical equipment has provided a powerful weapon for human beings to overcome disease problems. However, the safety hazard caused by insufficient attention to human factors engineering factors in the design process of medical equipment has been around for a long time. In addition, the use of medical equipment, materials, processing technology is constantly changing over time, product simplification has become a development trend, the use of medical equipment is constantly changing... It can be seen that the design and development of medical equipment still needs to overcome many difficulties. Therefore, in the day when design thinking is gradually complete and technology continues to improve, it is constantly learning lessons and gradually strengthening the regulation of human factors engineering [1] in the process of designing and developing medical equipment, becoming the key to seeking breakthroughs in medical products .
1. How to achieve equipment compliance + clinical service effectiveness two-way improvement?
How can we design a medical product suitable for users? Human factors engineering is one of the key considerations in the development of medical products, and the importance and necessity of its concept in the design and development of medical equipment is increasing. Therefore, this concept has begun to attract widespread attention in various medical device R&D companies and evaluation organizations.
In the past two years, China and Europe and the United States have once again strengthened the weight of human factors engineering and usability in the adjusted medical regulations, especially for medical devices that require an interactive interface and established steps, which require extremely high precision and reliability. . For example, in the revised ISO13485 medical equipment quality system in 2016, it was clearly stated in the specification requirements that human factors engineering should be included in the design input [2], and related fields also involve the output of demand, for example, in the expected environment of the product. Provides patient training based on established operations and safe use. In the MDR/2017/745 [3] launched in Europe in 2017, the human factors engineering and usability factors mentioned in MDD are also emphasized: medical companies should maximize the possible risks from the ergonomics and use environment; Comprehensive consideration of the user's technical knowledge, experience, educational background, training and use environment of the corresponding equipment. The US FDA also puts forward the requirements for ergonomics, as shown in the following figure:
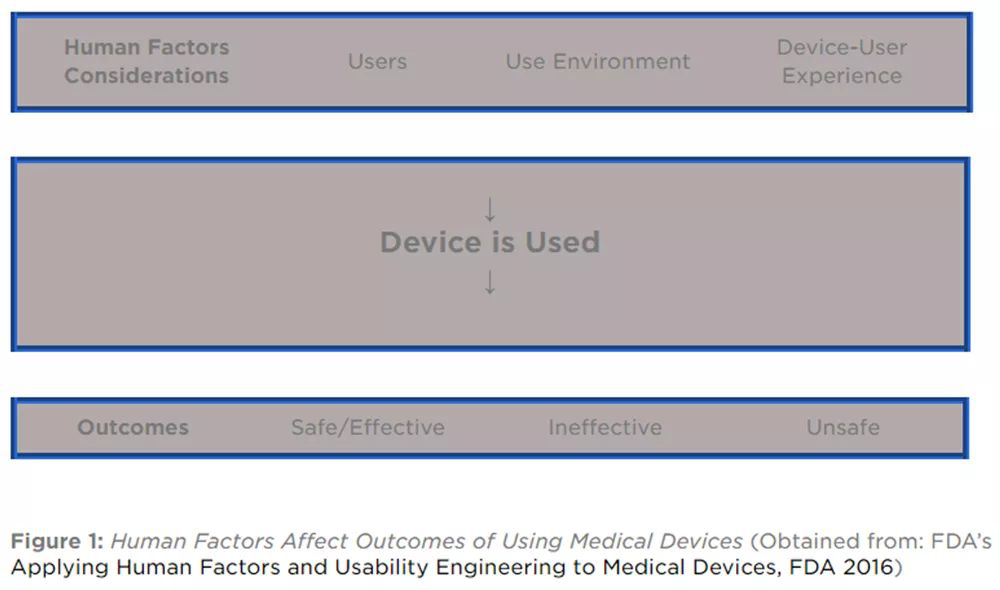
Image source: FDA2016
2. The significance of human factors engineering for medical equipment
Human factors engineering is a marginal applied science, which plays an important guiding role in the design and development of medical equipment. Human factors engineering combines the knowledge and results of disciplines such as physiology, psychology, medicine, hygiene, anthropometry, labor science, systems engineering, sociology, and management, through the proper design and improvement of human-machine-environment The interrelationship between the three makes the work system satisfactory, while at the same time ensuring people's safety, health and comfort.
Medical products that meet the human factors engineering requirements ultimately need to meet the following characteristics to ensure that the company meets both regulatory requirements and business objectives.
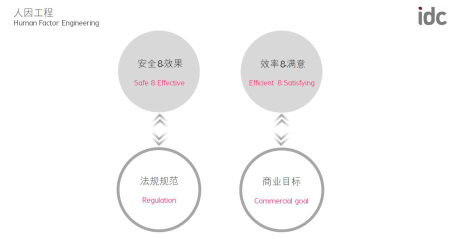
Product design goals achieved by human factors engineering
Dried Garlic Granules,Dried Granulated Garlic,Grated Garlic,Cream Dehydrated Garlic Powder
shandong changrong international trade co.,ltd. , https://www.changronggarliccn.com