
Clinical samples are treasures, don't let them sleep to death! ! !
In the process of communication with many doctors and teachers, I often receive such feedback: I have collected a lot of clinical samples, but I don't know how to use them. The more the samples are collected, the more the refrigerators are filled, but In the end, these samples have been sleeping, sleeping to death! In response to this, we recommend Jike's complete Biomarker screening program in this issue. Further development of kits, complete clinical transformation, benefit the people; step back can publish high score articles.
In July of this year, the Ministry of Science and Technology issued the “Guidelines for the 2017 Project Declaration Guide for the “Special Medical Research†Key Projectâ€, suggesting that “... establish a multi-level precision medical knowledge base system and biomedical big data sharing platform to break through the new generation of life. Group data analysis and clinical application technology, establish a large-scale disease early warning, diagnosis, treatment and efficacy evaluation of biomarkers, targets, preparations of experimental and analytical technology systems, the formation of a serious disease prevention and treatment plan and clinical decision-making system , Demonstration, application and promotion system for the construction of a clinical plan for the precise disease of typical diseases in China...".

The essence of precision medicine is the analysis, identification, verification and application of biomarkers for large sample populations and specific disease types through genomics, proteomics and other omics technologies and medical frontier technologies, so as to accurately find the cause and treatment of diseases. Targets, and accurately classify the different states and processes of a disease, and ultimately achieve the purpose of personalized and precise treatment of diseases and specific patients, and improve the effectiveness of disease diagnosis and treatment.
Biomarker refers to a biochemical indicator that can label changes or possible changes in the structure, function, organization, cell and subcellular structure or function of a system, and has a very wide range of uses. Not only can the pathogenesis be explored at the molecular level, but it also has unique advantages in accurately and sensitively evaluating early and low-level lesions. It can provide early warning, prognostic efficacy analysis, accurate disease staging classification, etc., to a large extent. It provides the clinician with a basis for assisted diagnosis. It is not difficult to see that Biomarker coincides with the concept of precision medicine and has a high degree of uniformity in the accurate diagnosis of diseases.

Using different omics techniques to search for Biomarker at different levels of different diseases, early diagnosis, prognosis, and classification through high-throughput screening, to accurately classify the different states and processes of the same disease, thereby on the basis of disease and Specific patient designs are personalized and precise treatment options. At this level, Biomarker is the premise of precision medicine. The precise Biomarker is very important for the subsequent implementation of precision medicine for different diseases. Therefore, the early screening and verification of Biomarker is particularly important.

In response to the screening and verification of Biomarker, Jikai integrates multiple platforms and develops a one-stop service. Based on clinical issues, sample and clinical data can be provided to complete the whole process from screening to verification to kit development. Process.
Clinical issues: Clinical issues are the core innovations of Biomarker screening. It can be the early diagnosis of disease, the efficacy analysis after drug administration, the disease classification judgment or the prognosis analysis. Any problem that can be raised in the clinic and can be sampled can be used as a foothold for Biomarker screening.
Clinical sample group accumulation and clinical data accumulation: Clinical samples are the basis of Biomarker screening. Therefore, while presenting clinical problems, it is necessary to consider the sample size that can be collected. From the early screening to the later verification, sufficient sample support is needed. The sample size is preferably more than 100 cases per year. The sample source is diverse. The basic principle is that non-invasive or minimally invasive samples are preferred. Whole blood, serum, plasma, urine, saliva, tissue, cerebrospinal fluid and even FFPE. As a screening sample. For grouping, there are at least control and experimental groups, and more rigorous programs often subdivide the experimental group and join the auxiliary group. In addition, clinical information is also very important, the patient's clinical data, pathological diagnosis results, follow-up records... These clinical information is the basis for the later establishment of the diagnosis Panel, which can greatly improve the reliability of Biomarker.
High-throughput screening and data analysis: Select the corresponding high-throughput screening method based on sample conditions and personal interests. From the group level, it can be a genome, a transcriptome or a proteome. From the screening method, it can be sequencing, chip, Mass spectrometry and more. The data obtained through high-throughput methods is very large, and it is necessary to have a strong bioinformatics team to analyze the raw data and extract valuable things (candidate Biomarker).
The medium and low-throughput technology expands the sample validation: After high-throughput screening and bio-sense analysis, the candidate Biomarker will be re-regressed into the clinical mid- and low-flux expanded sample validation. Different methods can be selected for Biomarker at different levels. Sequenom for SNP/SNV, qPCR for expression level, apparent marker, CNV, and ELISA, western and IHC for protein. In theory, the more the number of samples to be verified, the better, generally at least a hundred cases.
New test kit development: After obtaining Biomarker, bioinformatics analysis was used to establish a diagnostic panel, a clinical test kit was developed, and clinical tests were performed to verify the validity of the panel.
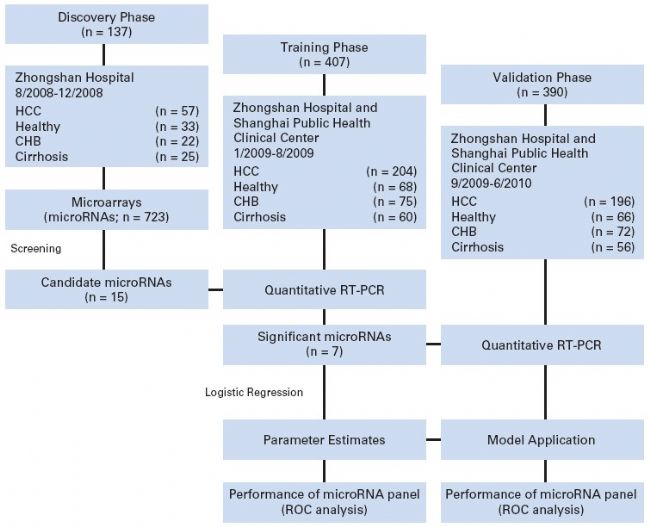
Due to limited space, a classic case of Biomarker screening is listed below: Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. This document was published in the Journal of Clinical Oncology in 2011 by Professor Fan Jia, a researcher at the Institute of Liver Cancer, Zhongshan Hospital, Fudan University. In this paper, Agilent microRNA chip screening and RT-PCR verification were used to screen the early liver cancer diagnostic molecular markers composed of 7 microRNAs in the plasma of HBV-related liver cancer patients. The diagnostic accuracy of HBV-related liver cancer less than 2 cm was close to 90. %, the effect is significantly better than the traditional detection method.

Analyze this document according to the Biomarker screening overall scheme. First, the proposed clinical problem is HBV-related early diagnosis of liver cancer with clear objectives. Second, the clinical sample is minimally invasively collected plasma, a good source of Biomarker, and detailed clinical information, except for the control group. In addition to the experimental group (HBV-related liver cancer patients), an auxiliary group of chronic hepatitis B and cirrhosis was added, which was designed to eliminate possible interference. Third, 15 differential miRNAs were screened by miRNA microarray. Fourth, using qPCR to perform low-throughput verification of 15 differential miRNAs in 407 samples, narrowing the target miRNA range to 7; Fifth, combined with clinical data, re-bioinformatics analysis, establish Panel, and then 390 The validity of Panel was verified in the sample. It was found that the use of the panel for early diagnosis of HBV-related liver cancer was as high as 94.1%. In addition, the accuracy of distinguishing between liver cancer and chronic hepatitis B was 84.2%, and the accuracy of distinguishing between liver cancer and liver cirrhosis was improved. Up to 88.4%.
Biomarker is the most direct and rapid diagnostic tool. Its screening and acquisition can play an important role in disease diagnosis, development, treatment, and efficacy monitoring. In recent years, finding and discovering valuable Biomarker has become an important hot spot in current research. Precision medicine is the future direction of medicine, including precise prevention, diagnosis, treatment and prognosis. The key to precision medical development lies in the discovery and clinical practice of Biomarker.
Teachers who have clinical samples on hand, hurry up and stop letting your sample sleep.

Peeled Black Garlic is peel them first, and then fermentation,It's easy to eat.
We know that garlic itself is a very good health food, while the role of black garlic is really amazing. For diabetes, hypertension, high cholesterol, cancer prevention and treatment have a very significant effect.
Once you have peeled the clove it is ready to eat.Black Garlic
should have 24 months shelf life if stored in the correct conditions.

Peeled Black Garlic,Peeled Fermented Black Garlic,Fresh Peeled Black Garlic,Organic Peeled Black Garlic
Zhucheng Tongxi Commercial And Trade Co.,Ltd. , https://www.blackgarlicgroup.com